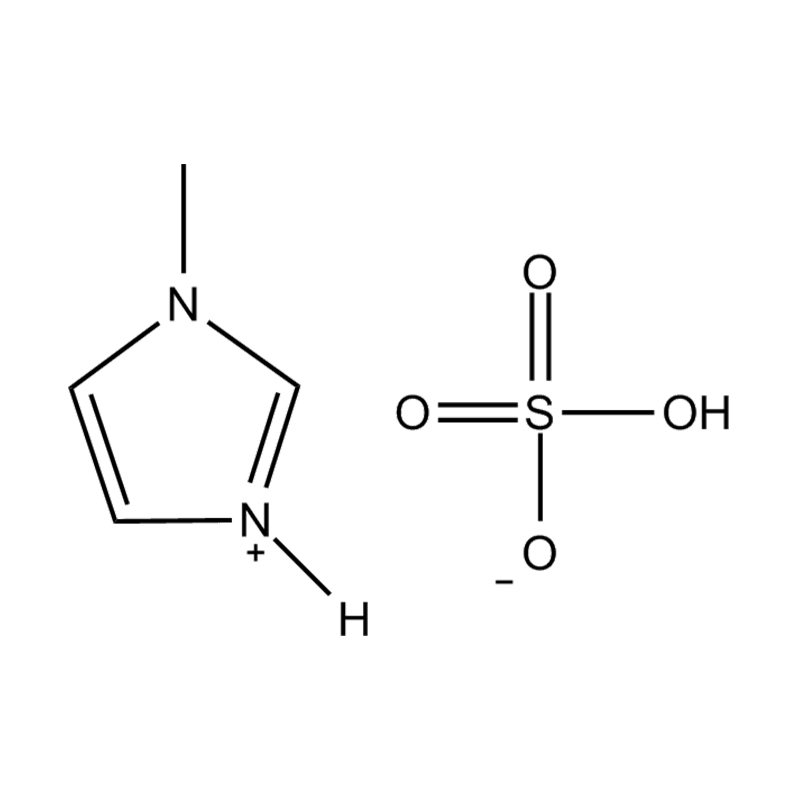
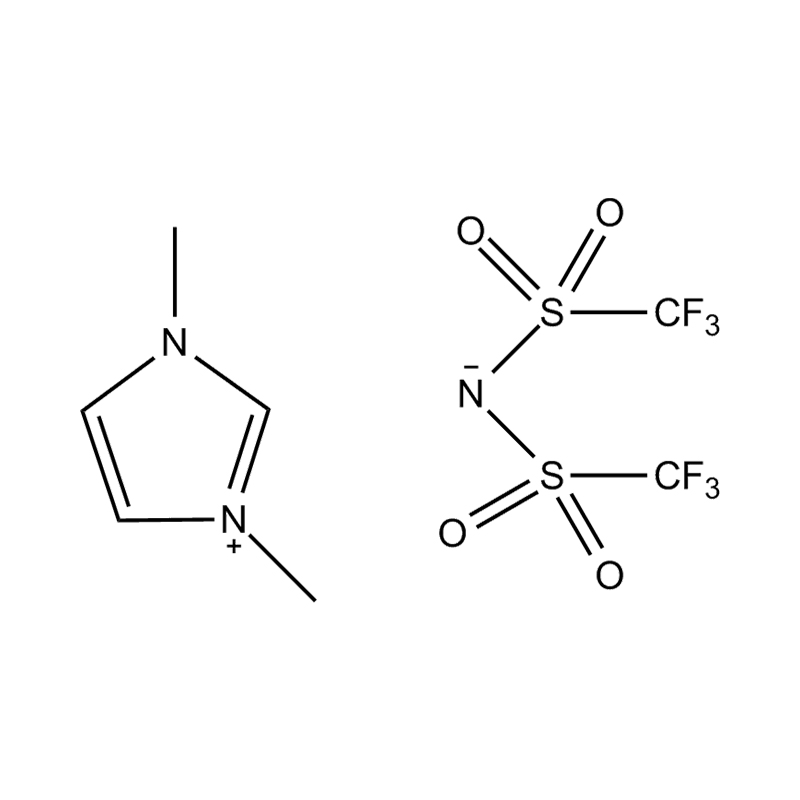
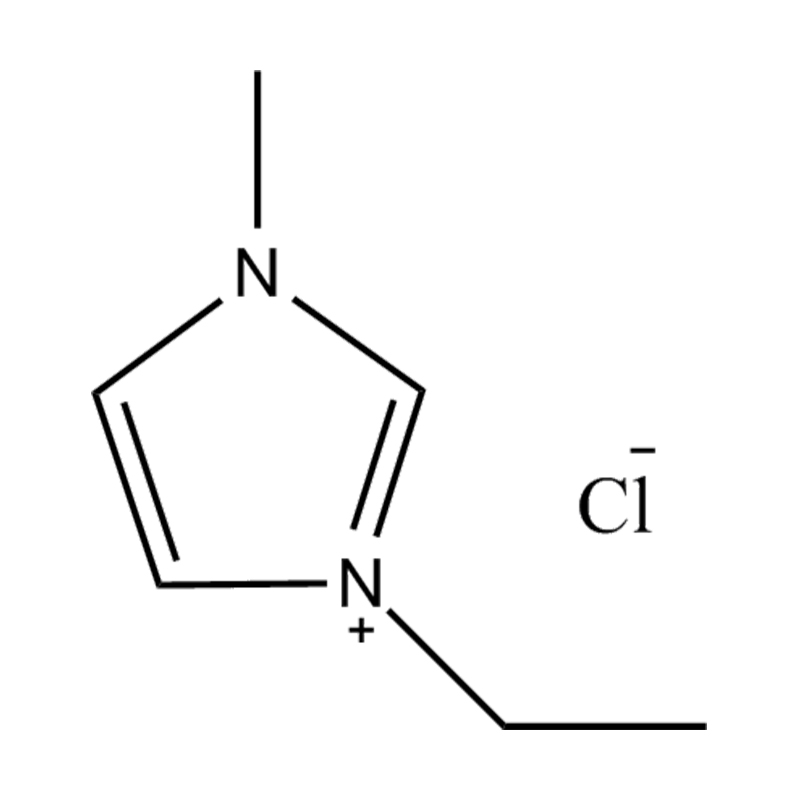
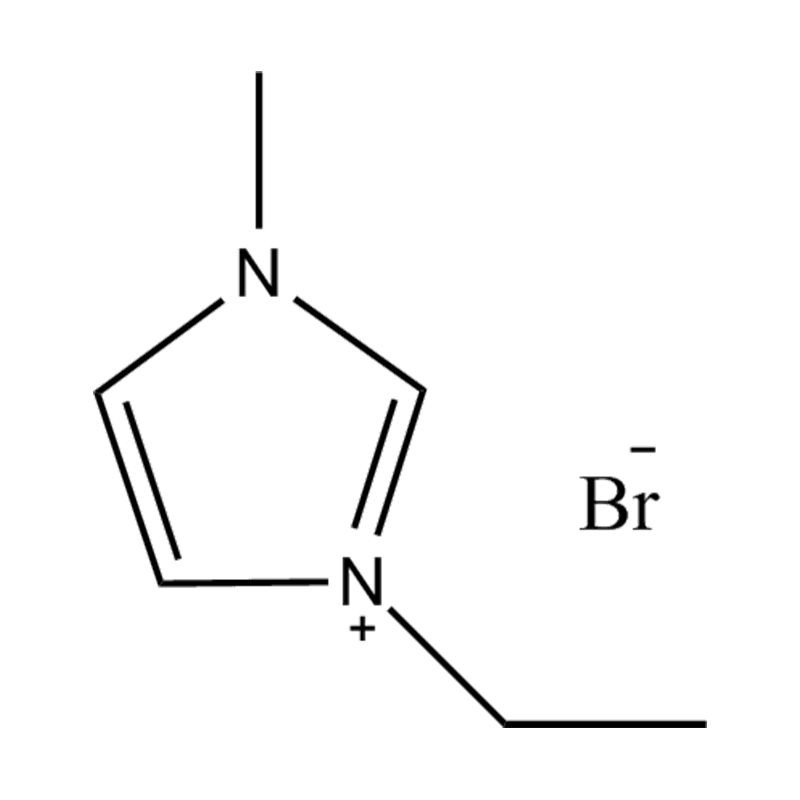
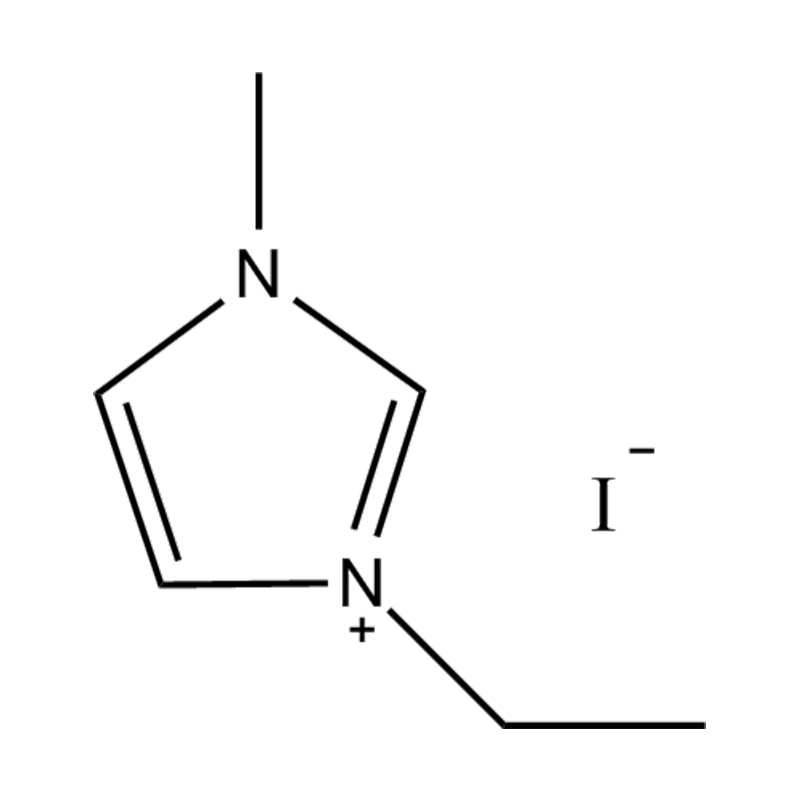
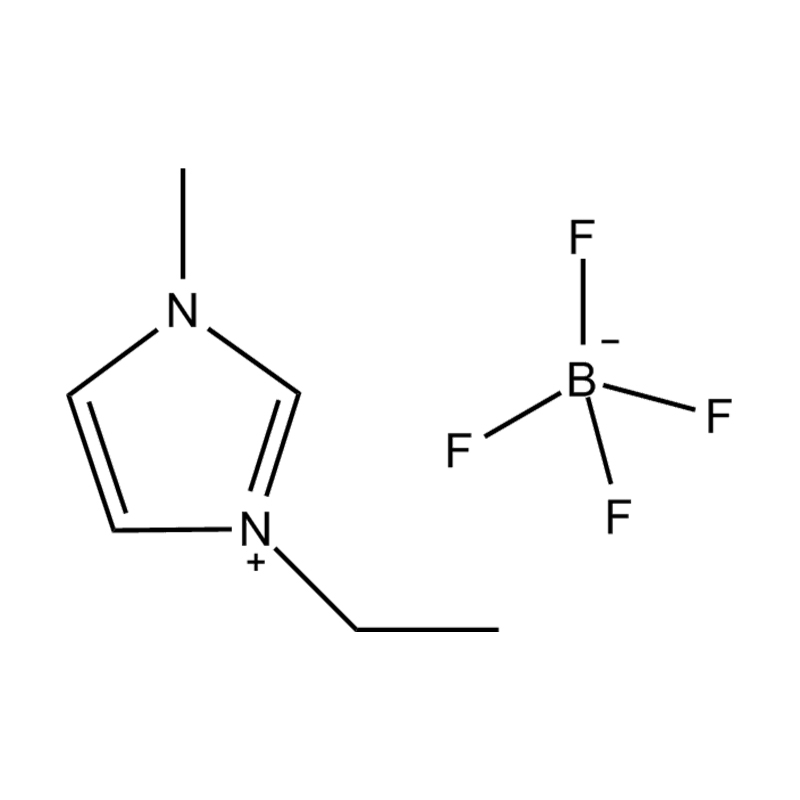
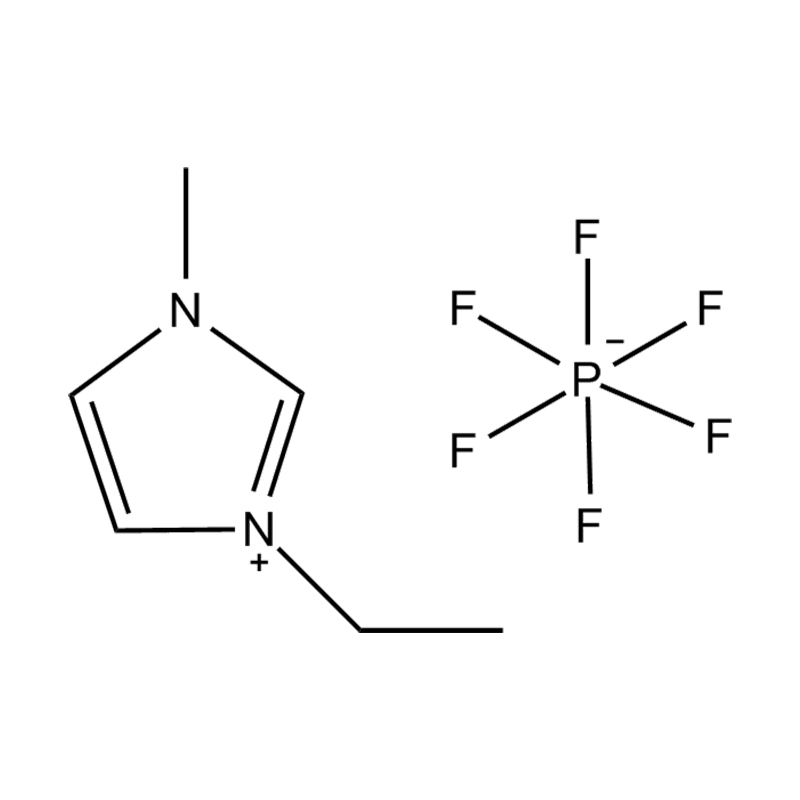
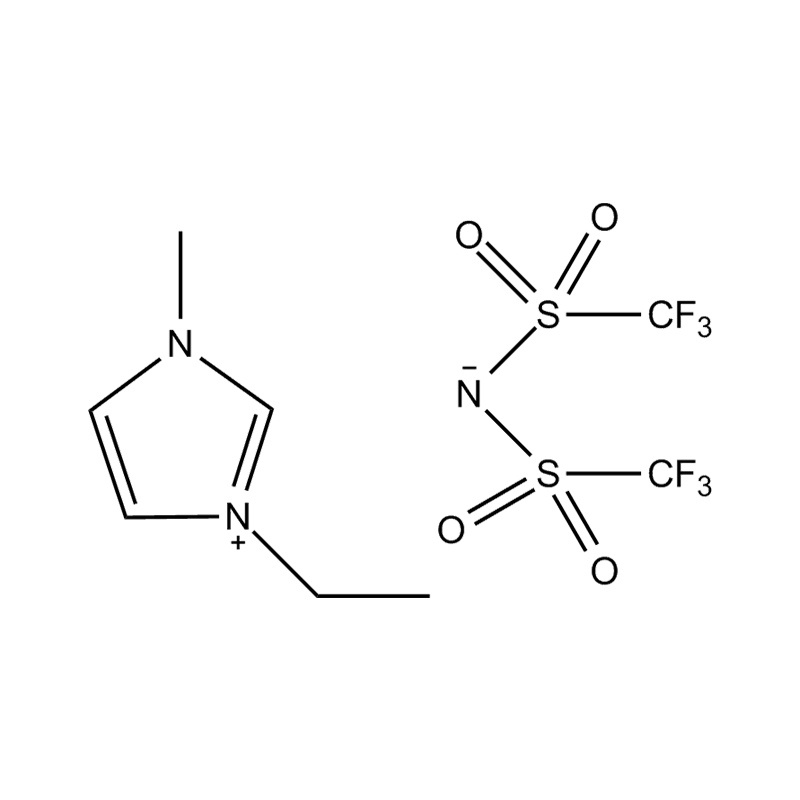
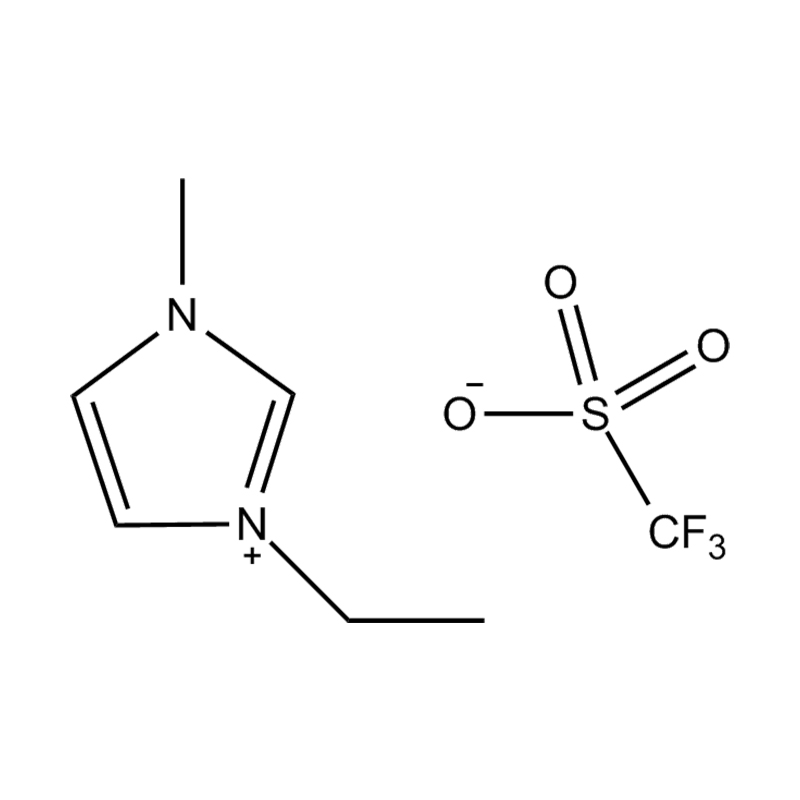
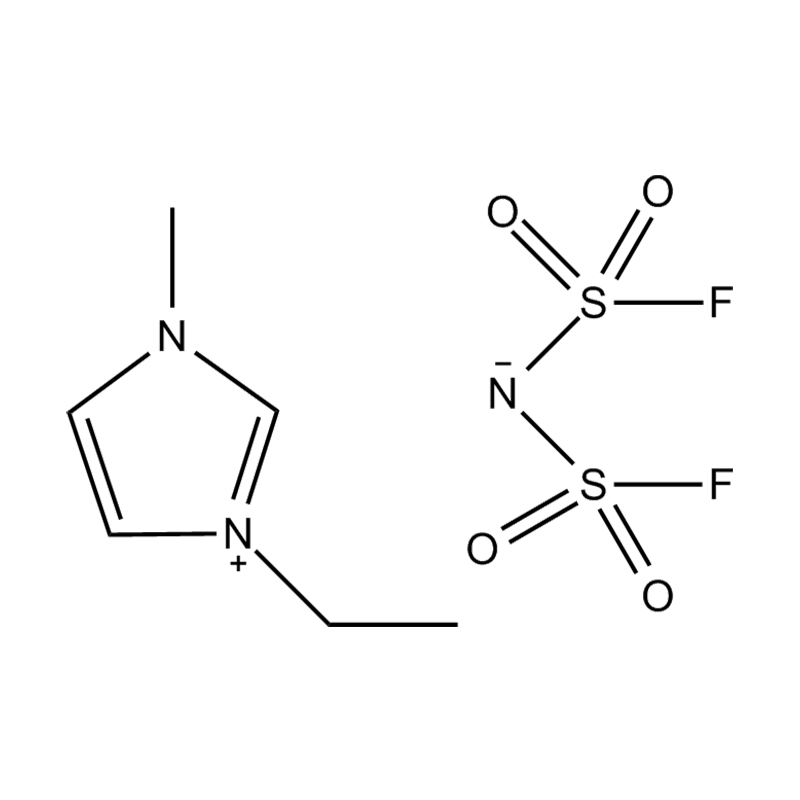
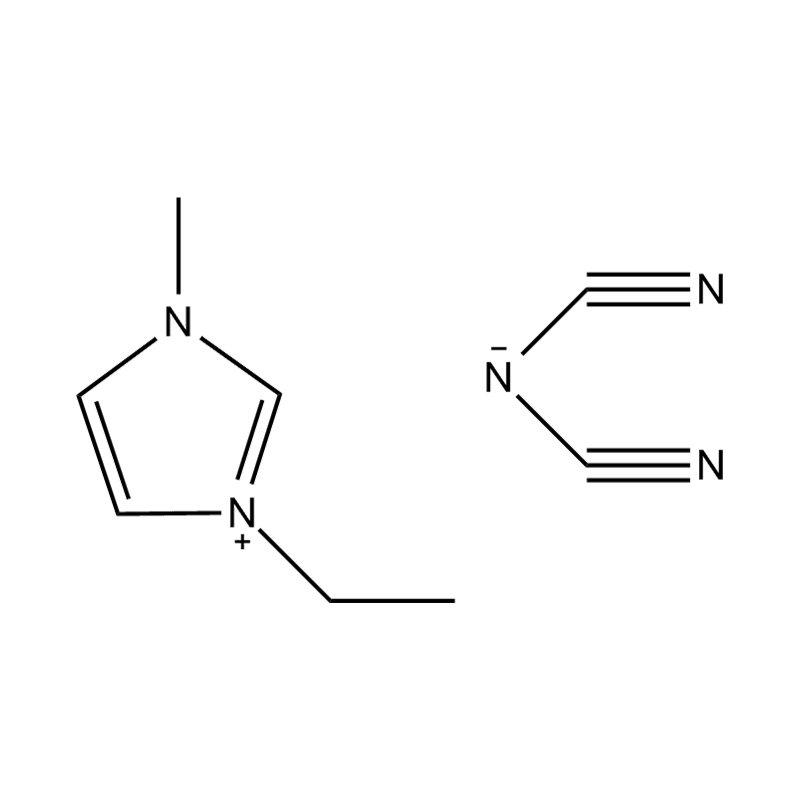
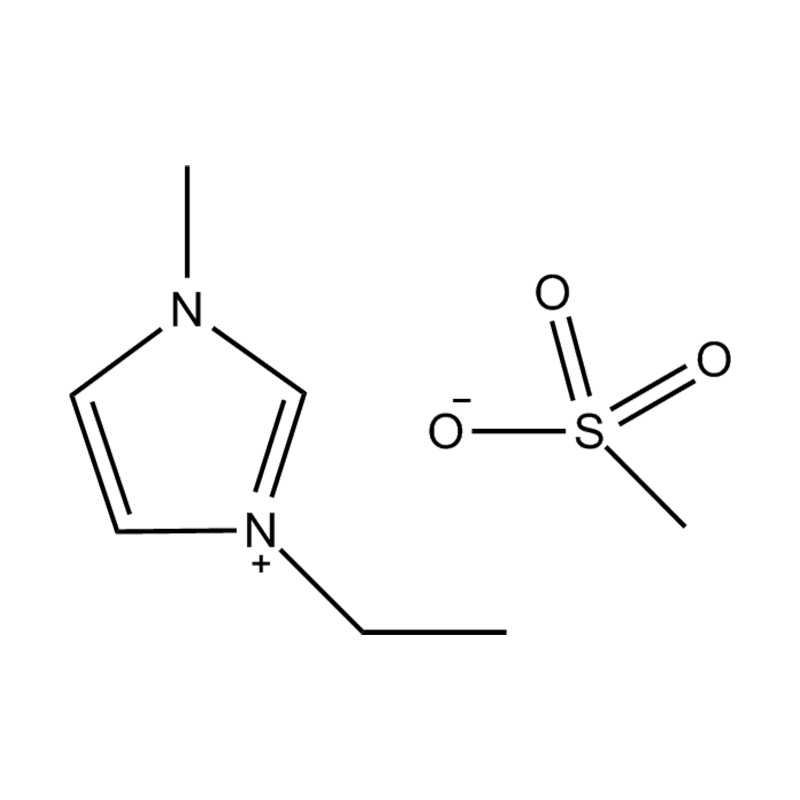
Disubstituted imidazole Ionicis liquida (Os) represents structuram tunable genus organicum sales, qui manent liquidum ad vel prope locus temperatus, distinguished a praesentia duorum substituent coetibus in imidazolium anulum. Hae componit offerre expansivum platform ad tailoring Ionic interactiones, physicochachem proprietatibus, et solvatio dynamics ad targeted applications per Catalysis, electromistry, materiae synthesis, et viridi elit. Hoc articulum DENDVS in Saccharum Strategies, structural-proprietas correlationibus, et eget deployment de Disubstituted imidazole ils, extollit partes in altera-generationem eget technologies.
I. Structural characteres et Saccharum meatus
Disubstitutio in imidazole anulus typically involves alkyl, Aryl, aether, aut heterocyclic substituents ad C2, C4, et C5 positions, ducens ad diversum electronic et steric effectus. Plerumque in N1 et N3 positions sunt officii cum alkyl aut heteroalkyl vincula, cum C2 positus est vel sinistra protonated vel electronice donando / subtractionis coetibus mutare hydrogenii moribus.
Synthesis plerumque per procedit per:
-
N-alkylation de imidazole Cum Halalalkanes cedere 1,3-Disubstituted imidazolium sales
-
Post-Functionalization Strategies, ut Quaternization, nucleophilic substitutione, aut metallation ad C2 loco
-
Anion commutationem processibus per metathesis vel acidum-basi reactiones ad introducendam non-coordinating vel munus-specifica anions (E.G .: [Pf₆] ⁻ [bf₄] ⁻ [ntf₂] ⁻, aut Halometallate Species)
Haec modifications critico influere clavis parametri ut scelerisque stabilitatem, hydrophobicity, viscositas, Ionic conductivity et coordinatio mores.
II. Physicochochemical proprietatio modulation
De physicochemical characteres de Disubstituted imidazole if maxime sensitivo utriusque cationic et anionic components. Per rationalem consilio, in sequentibus proprietatibus potest esse subtiliter accommodetur:
-
Viscosity et fluiditatem : Short-torquem alkyl substitutionibus typice reducere viscositas et augendae massa onerariam, cum longa vel rarissimis vincula crescat structural ordo et rheologica multiplicitate.
-
Thermal et electroChemical stabilitatem : Aromatophyta et bulky substituents potest amplio corrumpuntur temperaturis et expand electrochemical Fenestra, crucial pro altilium electrolytes et supercapacitori media.
-
Hydrophilicity / Hydrophobicity Libra : De natura anion et praesentiam Suspendisse Groups dictare aquam solubility et miscibility cum organicum solvents, impacting solvendo lectio in catalysis aut extraction.
-
Ionic conductivity : Auctus a reducendo Ion HYMENAEOS et augendae præcepta delocalization, typice per usum delocalized vel molestiam anions in combinatione cum minus coordinating cations.
Experimentalem techniques ut NMR, FTir, TGA, DSC, et dielectric Spectroscopy sunt petit usus ad resolvere haec proprietas et referunt ad Molecular architectura.
III. Solvatio et Hydrogenii Bonding MORES
Et unique facultatem de imidazolium, secundum, ut forma extensive hydrogenii vinculum retiacula, praesertim cum C2 hydrogenii retinetur, underpins eorum eximia solvit potentia. Disubitiones hujusmodi alters hydrogenii vinculum donatoris fortitudinem, ita modulating commercium cum solutes, reagentia et catalytic centra.
Computational Studies et IR spectroscopy revelare C2-fingitur ils exhibeatur reducitur verticitatem minui facultatem contristat solute solvendo interactiones, faciens idoneam ad selectivam solvendo tasks vel stabiliente laboris selectivam communicationem vel stabilientem laboris selectivam communicationem vel stabilientem laboris selectivam SOLVABRIS tasks vel stabilientem labores selectivam SOLVATIO tasks vel stabilientem laboris selectivam Solvat tasks aut stabilientem labores selectivam Solvat tasks vel stabilientem laboris selectivam in organicum synthesim.
IV. Applications contra scientific domains
Et versatility of Disubstituted imidazole ii patet per eorum expanding partes in utroque fundamentalis et applicari investigationis:
A. Catalysis et reactionem media
Hi servio sicut non-volatile, thermally stabilis media ad transitus metallum catalysis, bruantur / Lewis Acidum Catalysis et Biocatysysis. Electronically modified imidazolium, potest stabiliendum reactive intermedia aut serve ut co-catalysts, praecipue in carbon-ipsum coupling reactiones, cycloadditions, aut oxidative processibus.
b. Electrochemical cogitationes
In High Ionic conductivity et scelerisque stabilitatem, Disubstituted imidazolium, sunt idealis pro electroChemical applications comprehendo:
-
Lithium, Ion et sodium-Ion altilium electrolytes
-
Supercapacitor Media cum Wide Electrochemical Fenestra
-
Electroplatting balneis pro metallis ut al, Zn, aut rara terrae
c. Separation scientia et extraction
Sailor-fecit, cum propria polarity et affinitatem characteres potest adhiberi in liquido liquido extractionibus, Gas effusio (E.G., CO₂ capere), et separationem mixturis, rara metalla, aut azeotropic mixturis.
d. Materias Chemiae et Nanotechnology
Paulables agentibus agentibus, solvents, aut superficiem modifiers in synthesim Nanostructured materiae, inter metallum-organicum frameworks (MOFS), Nanoporous carbons et cadmiae Nanomaterials. Eorum non-volatile et Suspendisse environment sustinet precise imperium super nucleation et incrementum dynamics.
V. Aliquam et Toxicological Consid
Non obstante eorum viridi chemistry fama ut non-volatile alternatives ad organicum solvents, in environmental profile of imidazole ils requirit diligenter aestimationem. Disubstituted variants, praecipue illis cum longa alkyl vincula vel halogenated anions, ut exhibeant persistentiam, bioaccum ullo potentiale, aut aquatilium toxicity.
Recent Developments Focus in:
-
Designing biodegradable ils Using Esther, Aide, aut sugar, derived substituents
-
Switchable Polarity Systems Ad facilitate recuperatio et reuse
-
Toxicity reductionem per Anion Optimization Et non-halogenated alternatives sicut alkyl sulfate aut amino acid-fundatur anions
VI. Future directiones et investigationis challenges
Progressi ad utilitatem de Disubstituted imidazole Ionic liquores involves aliquot clavem provocationes:
-
Predictive modeling structuram proprietas relationes , Per apparatus doctrina et quantum chemical calculations
-
Integration in muneris materiae ut Polymer-II composita, Ionogels, aut liquido sustinetur
-
Scalable et sumptus-effective synthesis , Praesertim ad Industrial-gradus applications
-
Vita-Cicero analysis et regulatory obsequio Ut sustineri exsequendam
Disubstituted imidazole, secundum Ionic liquores repraesentant modulari et function-dives ordinis de componit capaces capax loca multa scientific disciplinis. Per leveraging prostisise mulgare ipsum, investigatores potest reserare amplis et chemical partum tailored ad emergentes eget in viridi chemica, industria repono, et provectus vestibulum. Continued nisus in rationali consilio, environmental taxationem, et applicationem-repulsi research erit essentialis ad animadverto eorum plenus potential in sustineri eget technologies.



 中文简体
中文简体