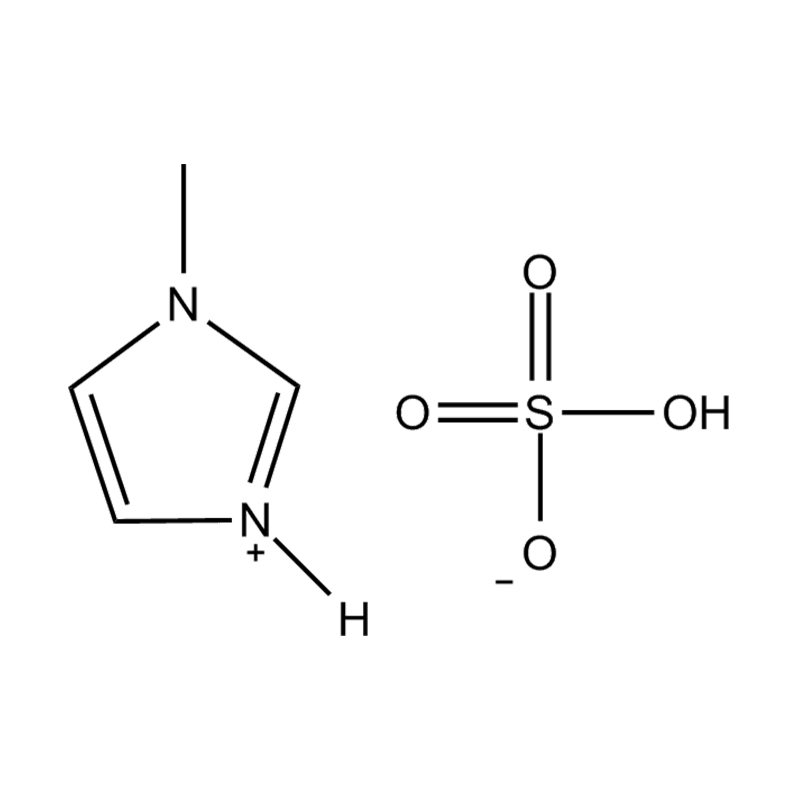
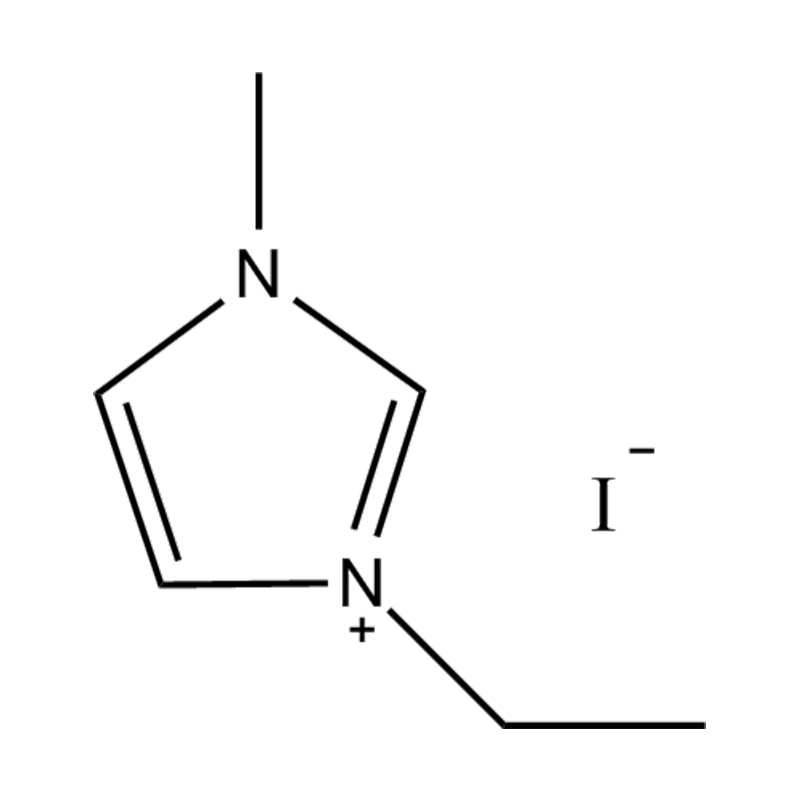
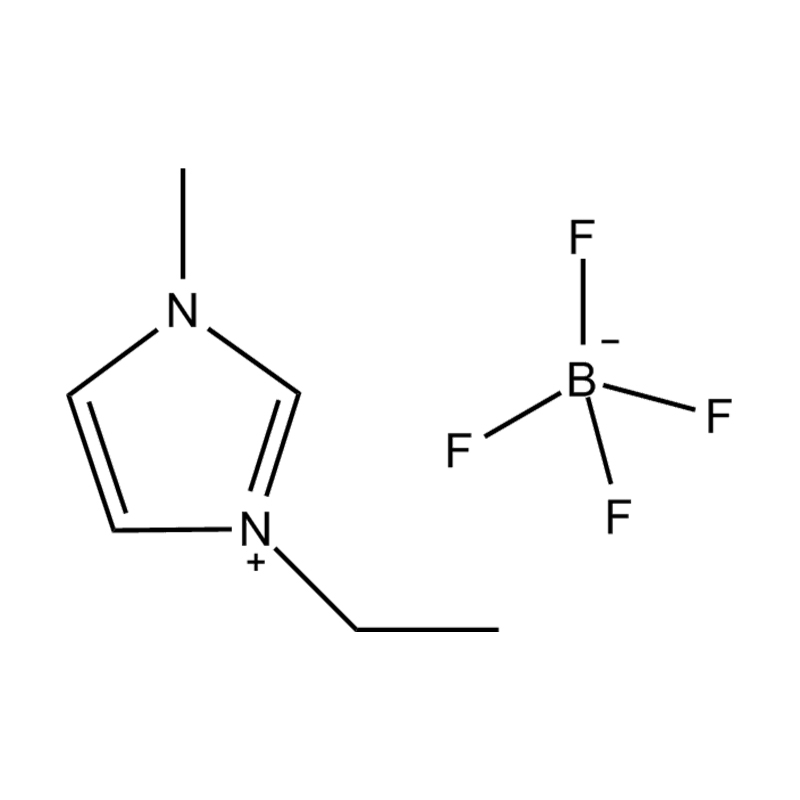
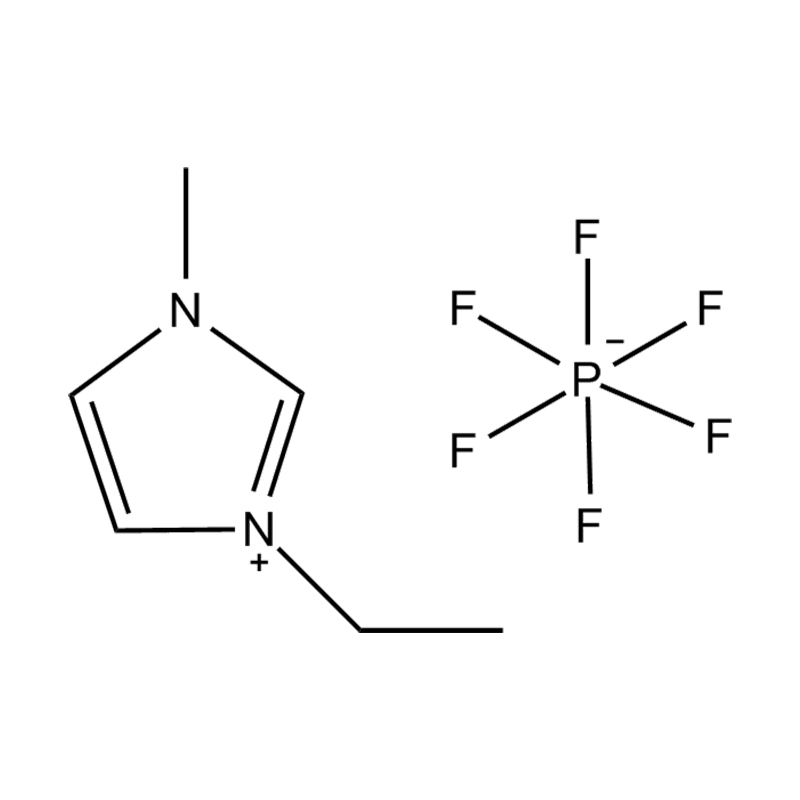
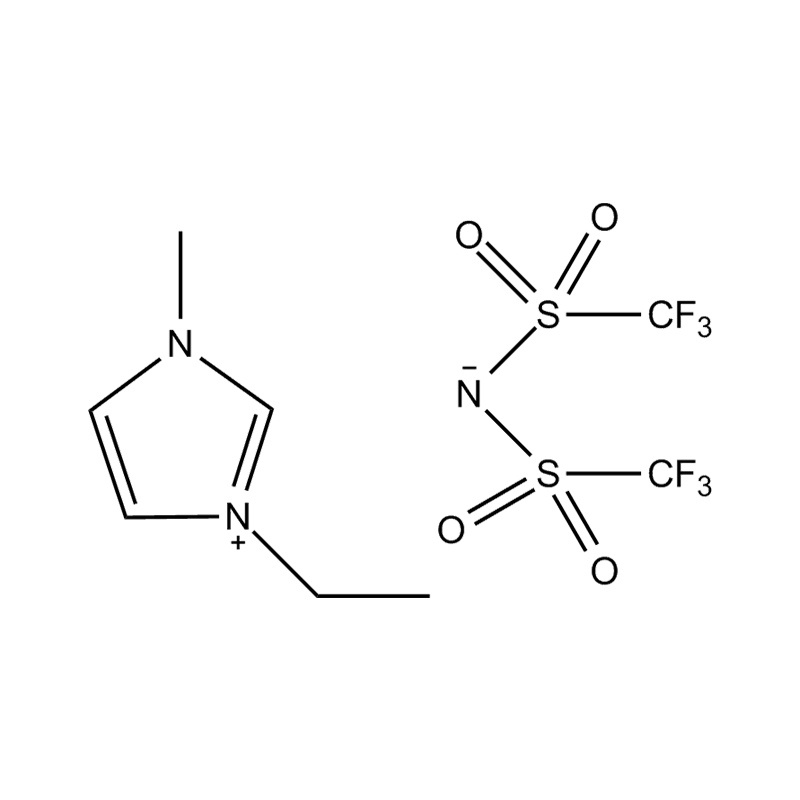
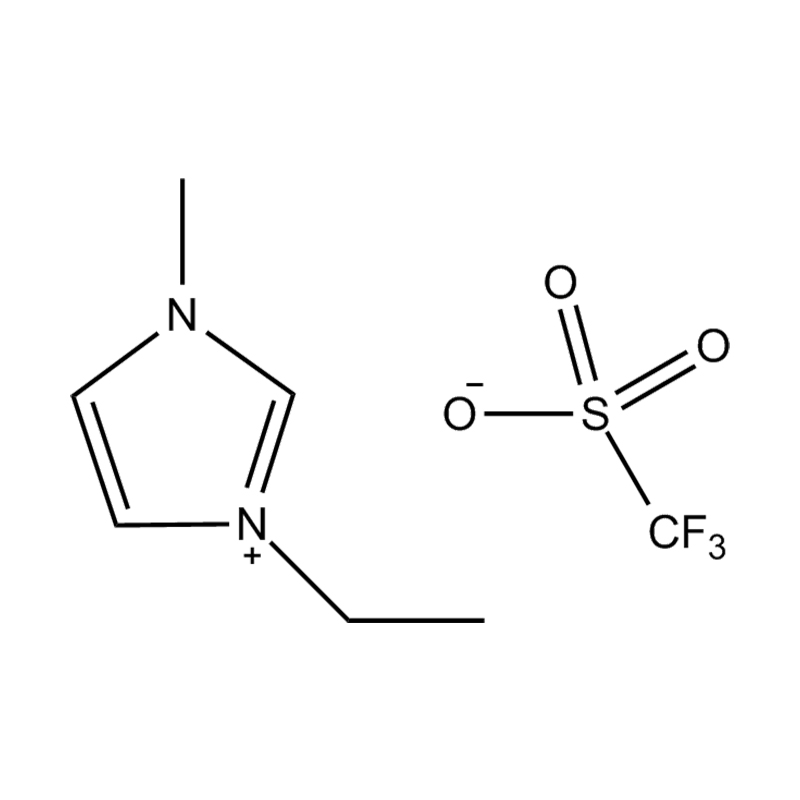
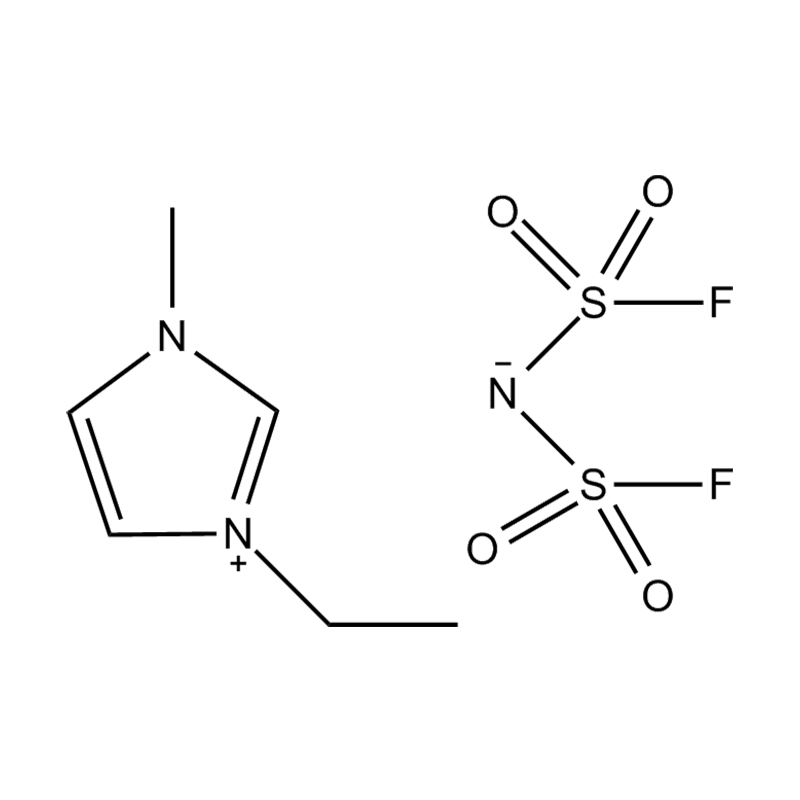
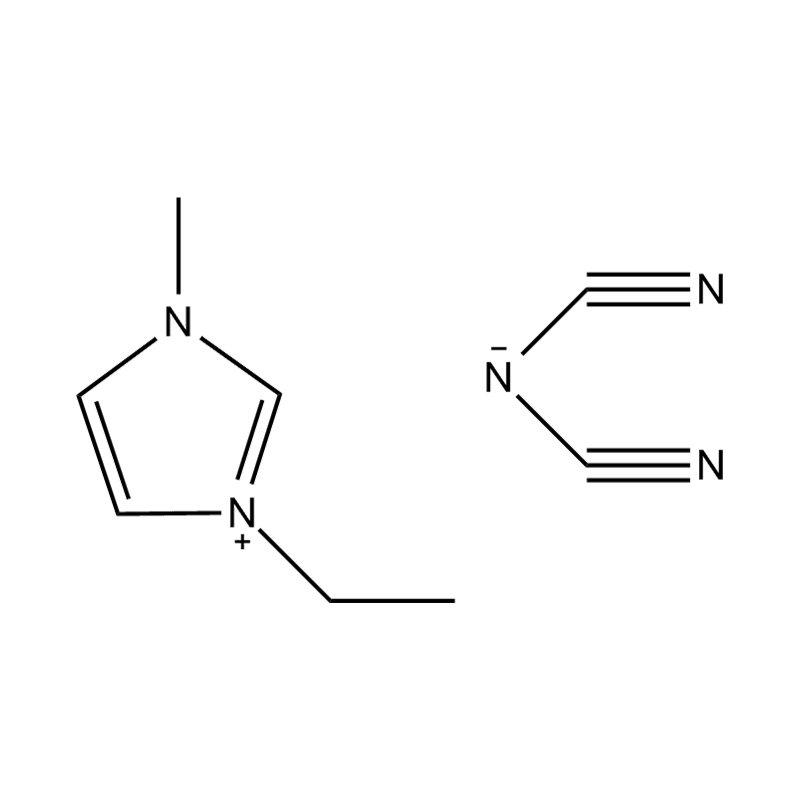
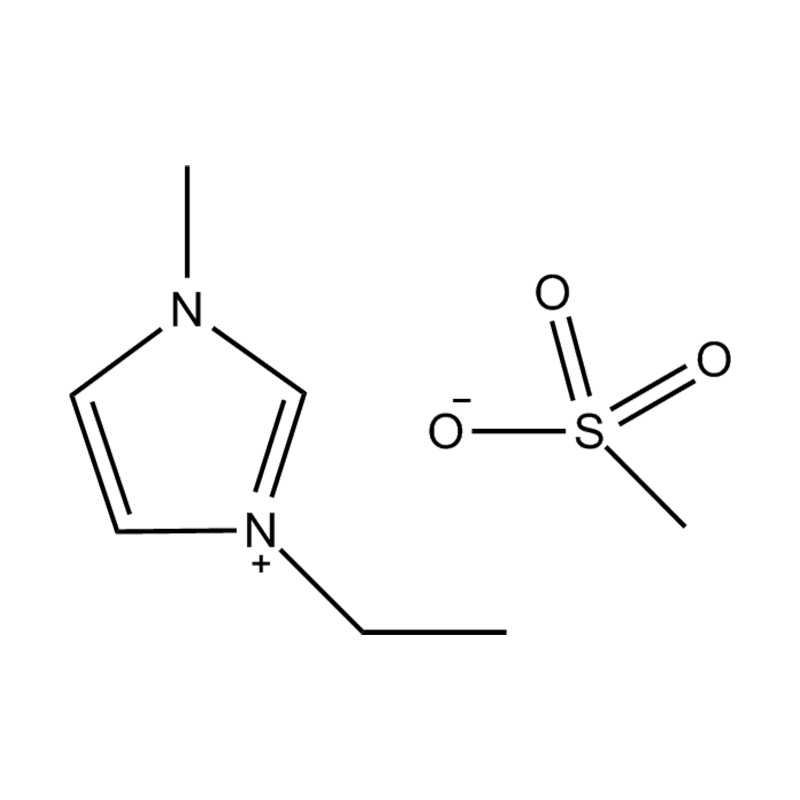
Et electrochemical stabilitatem Disubstituted imidazole Ionicis liquida In alto voltage vel redox-activae environments influere pluribus inter se mechanisms radicatur in mocecular structuram et electronic configuratione:
Electron Delocalization in Imidazole Ring: De natura Aromaticum de Imidazole Orbis sino pro significant Delocalization de Π-electrons, quod enhances in moleculo resistentia ad oxidative vel reductive degradation. Cum substitutis ad utrumque 1- et III, positions, electronic densitas potest redistributed in via, quae stabilit in catione contra electronicam translationis reactiones.
Substituent effectus, quod genus et positus substituents in imidazole annulum signanter afficit electroChemical stabilitatem. Electron-donando coetus potest augendae nucleophilicity et reducere oxidative stabilitatem, cum electronic, receditur coetus (ut halogens et nitriles) potest amplio oxidative resistentia per stabilientem summum occupata Molecular (homo). Vice versa, haec coetibus potest etiam inferior reductionem potentiale per stabilientem lowest unoccupied Molecular orbitalis (Lumo), fretus elit.
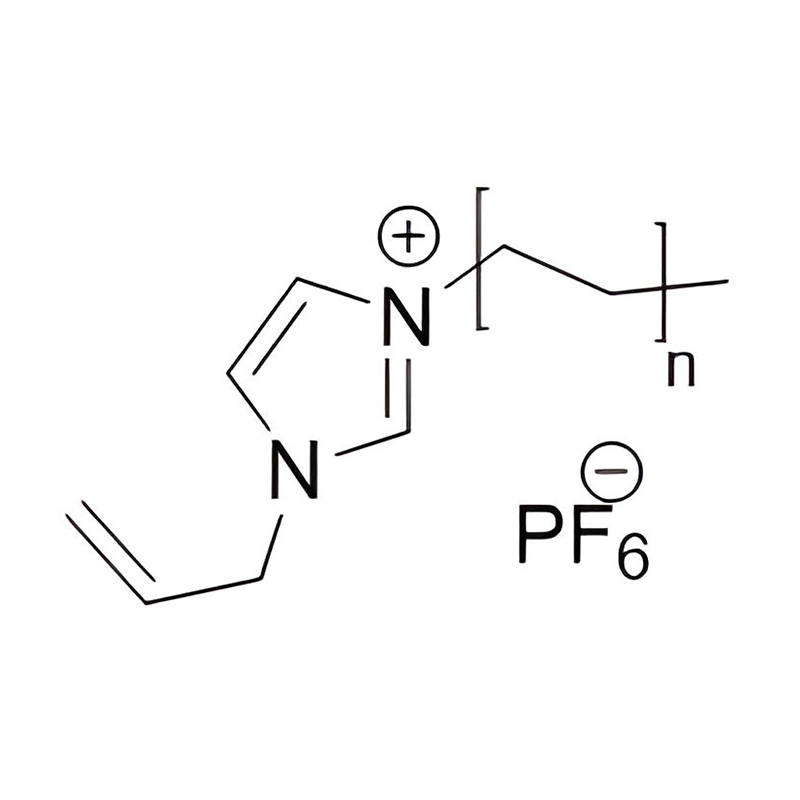
Steric impedimentum et loci scutum, bulky substituents ad 1- et III, positions potest physice clypeus in imidazolium circulum ex nucleophilic vel electrophilic impetum, limitando unwanted lateribus, quae posset fieri sub alto voltage condiciones.
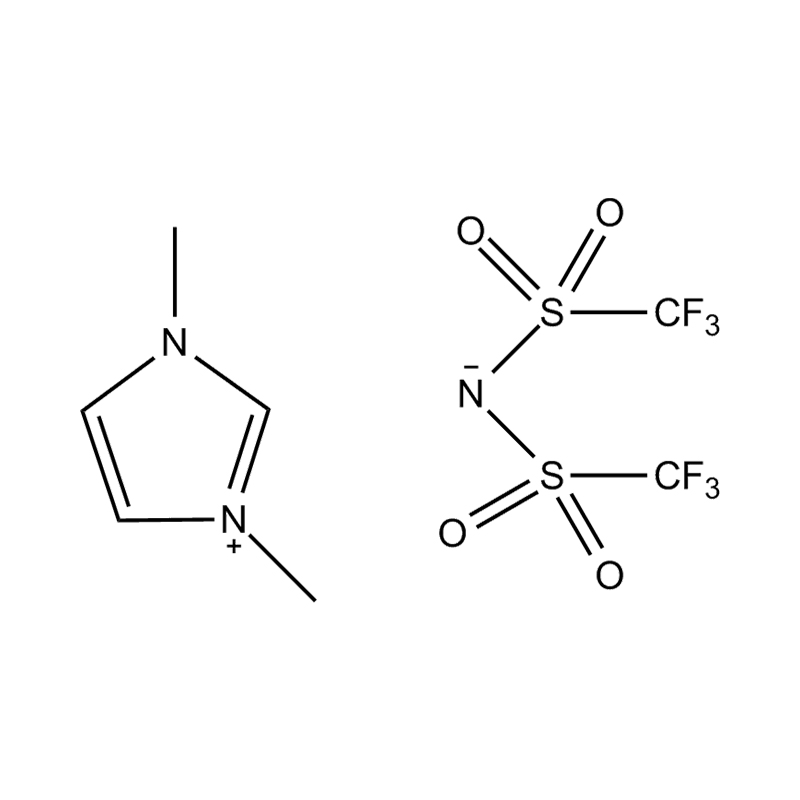
Stabilitatem autem anion-cation par, in HYMENAEOS et de Disubstituted imidazolium cationem cum stabilis, non-coordinantes anion (E.G., bis (TFSI⁻] vel tetrafluorol [bfs]) vel tetraflelormical fenestra. Hae anions resistite corrumpuntur et ponere Ionic conductivity sine intercedendo in redox reactiones.
Ion mobilitatem et interfacial behavior, in altus-voltage systems, praecipue in electrochemical cogitationes, mobilitatem ions et organization ad electrode interfaces influentiam stabilitatem. Disubstituted imidazole Ionicis liquida ut formare bene ordinata interfacial stratis, quod ne recta electrode translatio electrode et Ionic speciei, enhanced eorum electrochemical fenestra.
Thermal stabilitatem et corrumpuntur meatus: Quod intrinseco scelerisque stabilitatem de disubstitutus imidazole structuram minimizes periculum scelerisque corrumpuntur sub electroChemical accentus, quod est saepe cum voltage-adductus degradation.



 中文简体
中文简体